Background
Isocitrate Dehydrogenase (IDH) 1 or 2 mutations occur in ~20% of acute myeloid leukemia (AML). Both venetoclax (VEN)-based and targeted IDH-inhibitor (IDHi) therapies are effective treatment options for IDH mutated AML in combination with hypomethylating agents (HMA). ASTX727 is an oral, fixed dose (35 mg/100 mg) combination of decitabine and cedazuridine with bioequivalency to IV decitabine. Herein we report interim results including completion of the Phase 1b portion of the first all-oral triplet regimen of ASTX727 (day 1-5) + VEN (day 1-14) in combination with a targeted mutant IDH1i ivosidenib (IVO) or IDH2i enasidenib (ENA), for IDH mutated AML.
Methods
Eligible patients (pts) were > 18 years old with newly diagnosed (ND) AML not eligible for intensive chemotherapy, or relapsed/refractory (R/R) IDH1 or IDH2 mutated AML. Prior VEN, HMA, and/or IDHi use was not exclusionary in the R/R cohort. The primary objectives were to determine safety and the recommended phase 2 dose (RP2D) of ASTX727 and VEN with IVO (Arm A) or ENA (Arm B) [Phase 1b], and to determine the composite remission rate (CRc; CRh+CRi+CR) for both arms [Phase 2].
Results
A total of 51 pts (1b: 26 pts, 2: 25 pts) have been enrolled. The phase 1b portion is completed and the RP2D is established at VEN 600mg in combination with IVO (due to the CYP3A4 inducing effects of ivosidenib requiring a higher dose of VEN to maintain therapeutic concentrations), and VEN 400mg in combination with ENA.
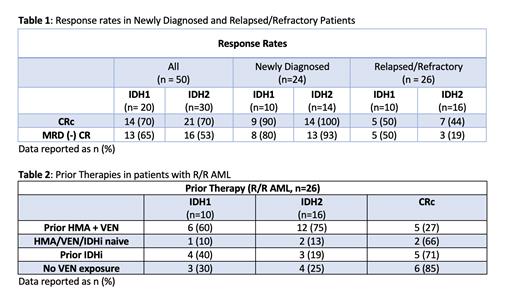
There are currently 50 evaluable pts (Arm A: 20, Arm B: 30); median follow up is 11.0 months (mos) for ND pts and 14.6 mos for R/R pts. Median age at enrollment was 72 years (41 - 86). 48% (n=24) pts had ND-AML (A: 10, B: 14) and 52% (n = 26) with R/R AML (A: 10, B: 16). ELN 2022 classification was intermediate or adverse in 87% of ND-AML and 92% of the R/R AML cohorts. Median variant allele frequency in ND pts was 22% (7-38, IDH1) and 36% (4-49, IDH2) and was 29% (8-45, IDH1) and 37% (2-48, IDH2) in the R/R arms. Patients with R/R-AML received a median of 2 lines of prior therapy, including 20 pts having received prior VEN and 7 receiving prior IDH-inhibitor.
The composite remission rate (CRc), defined as CR + CRh + CRi, was 96% (n=23) in ND-AML and 46% (n=12) with R/R disease (Table 1). Of pts who received prior VEN, 30% (n=6) achieved a CRc (3 CR, 2 CRi, 1CRh) and in those with prior IDHi exposure, 71% (n=5) achieved a CRc (4 CR, 1 CRh)(Table 2). Measurable residual disease (MRD) negative CRc by flow cytometry was achieved as best response in 91% of the responding ND pts (35% IDH1 and 56% IDH2) and 67% (42% IDH1 and 25% IDH2) of R/R pts. Duration of response (DOR) was not reached (NR) for ND pts and 16.1 mos in R/R pts. Event free survival (EFS) was NR and 5.9 mos (2.76 - NA), respectively. Overall survival (OS) was NR for ND and 10.4 mos (4.7 - NA) for R/R AML.
The most common non-hematologic grade 3/4 AEs included indirect hyperbilirubinemia (n=3, 6%) all in enasidenib-treated pts, and two pts (4%) had grade 3 mucositis, one which was attributed to prior cytoreductive therapy. AEs of special interest regardless of grade included differentiation syndrome in 10% (n=5, A:3; B:2), all successfully managed with corticosteroids and diuresis. 4% (n=2) had TLS which was medically managed, one pt required CRRT briefly with full recovery of renal function. 19 pts remain on study, 8 transitioned to SCT in response, and 5 pts (4 ND, 1 R/R) withdrew from the study, including 4 in CRc response, after 2 (n=2), 5 (n=1) and 8 (n=1) cycles of protocol therapy.
Conclusions
Triplet therapy with ASTX727 + VEN + IDH1/2 inhibition appears safe and preliminarily effective for IDH mutated AML, with high rates of MRD-negative CRc particularly in ND pts and in R/R pts not having received prior VEN or IDHi. AEs were anticipated and tolerable. Enrollment is ongoing.
Disclosures
Loghavi:Gerson Lehrman Group: Consultancy; Daiichi Sankyo: Consultancy; QualWorld: Consultancy; Recordati/ EUSA Pharma: Consultancy; Guidepoint: Consultancy; Caris Diagnostics: Consultancy; Blueprint Medicine: Consultancy; Abbvie: Consultancy; Astellas: Research Funding; Amgen: Research Funding; Abbvie: Current equity holder in publicly-traded company. Maiti:Celgene: Research Funding; Lin BioScience: Research Funding. Daver:Gilead: Consultancy, Research Funding; Servier: Consultancy, Research Funding; AROG: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy; AbbVie: Consultancy, Research Funding; Agios: Consultancy; Pfizer: Consultancy, Research Funding; Glycomimetics: Research Funding; Jazz: Consultancy; Syndax: Consultancy; Trovagene: Research Funding; Trillium: Consultancy, Research Funding; FATE: Research Funding; Celgene: Consultancy; Kite, a Gilead company: Consultancy, Research Funding; Shattuck Labs: Consultancy; ImmunoGen: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Novimmune: Research Funding; Genentech: Consultancy, Research Funding; Hanmi: Research Funding; Astellas: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Kronos Bio: Research Funding. Alvarado Valero:Astex: Research Funding; Sun Pharma: Consultancy, Research Funding; Daiichi-Sankyo: Research Funding; Jazz: Research Funding; MEI Pharma: Research Funding; FibroGen: Research Funding; BerGenBio: Research Funding; CytomX Therapeutics: Consultancy. Pemmaraju:PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASCO Cancer.Net Editorial Board: Other: Leadership; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; United States Department of Defense (DOD): Research Funding; Karger Publishers: Other: Licenses; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend:: Membership on an entity's Board of Directors or advisory committees. Chien:Rigel Pharmaceuticals: Consultancy; AbbVie: Consultancy. Ferrajoli:GenMab: Research Funding; Beigene: Research Funding; AstraZeneca: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Genetech: Honoraria; Janssen: Honoraria. Short:Takeda: Consultancy, Research Funding; Pfizer: Consultancy; AstraZeneca: Consultancy; Novartis: Consultancy; Stemline therapeutics: Research Funding; Astellas: Research Funding; Amgen: Honoraria. Jabbour:Adaptive Biotech: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding. Andreeff:PMV: Research Funding; Kintor Pharmaceutical: Research Funding. Ravandi:Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Prelude: Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Biomea fusion: Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Syros: Consultancy, Honoraria, Research Funding. Konopleva:AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy; Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding; Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties. Garcia-Manero:Genentech: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding; AbbVie: Research Funding. Kantarjian:Daiichih-Sankyo (Inst): Honoraria, Research Funding; Immunogen (Inst): Honoraria, Research Funding; Ipsen: Honoraria; Jazz Pharmaceuticals (Inst): Honoraria, Research Funding; KAHR Medical: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Precision Biosciences: Honoraria; Shenzhen Target Rx: Honoraria; Abbvie (Inst): Research Funding; Taiho Pharmaceutical: Honoraria; Amgen (Inst): Research Funding; Ascentage Pharma (Inst): Research Funding; Bristol-Myers Squibb (Inst): Research Funding; Novartis (Inst): Research Funding; AstraZeneca/MedImmune: Honoraria; Astellas Pharma: Honoraria; Ascentage Pharma Group: Honoraria; Amgen: Honoraria; Abbvie: Consultancy, Honoraria. DiNardo:ImmuniOnc: Honoraria; Servier: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Fogham: Honoraria; AbbVie/Genentech: Honoraria; Schrödinger: Consultancy; Notable Labs: Honoraria; Astellas: Honoraria; BMS: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal